GlaxoSmithKline shares were at a peak of 1,846p just before the pandemic began in January 2020, since then GSK had followed a downtrend up until March 2021.
Economies around the world faced a slowdown in March 2021, which would explain the drop in GlaxoSmithKline shares as well. However, since then the GSK share price has been in uptrend.
GlaxoSmithKline’s CEO did say in their FY21 results that 2022 will be a “landmark year” for GSK but will it cross the 1,900p mark?
GSK Results:
In the first quarter of 2022, GlaxoSmithKline recorded a 32% increase in revenue to £9.7bn with Commercial Operations contributing £7.1bn.
Specialty Medicines brought in £3.1bn, accounting for nearly half of the £7bn contributions from Commercial Operations. The category grew by 98%, to sustain an increase across all therapy areas, including Xevudy sales. GlaxoSmithKline received £1.3bn on the sale of Xevudy.
Vaccine turnover increased by 36% to £1.66bn, led by Shingrix which is a shingles vaccine for adults aged 50 and over, in the US and Europe, reflecting robust performance and the advantage of a favourable comparative in Q1 2021, when sales were hit by COVID-19-related disruptions in numerous markets and decreased Centers for Disease Control purchases.
Thanks to Trelegy’s growth in all regions, the antibiotics market’s recovery, and the benefit of a favourable prior period returns and rebates adjustment, which helped to offset the impact of generic competition in the US, Europe, and Japan, GlaxoSmithKline’s General Medicines generated £2.34bn, up 2%.
Consumer Healthcare accounted for the remaining £2.6bn of the group’s revenue, which increased by 14%.
According to the company’s demerging plans, GSK consumer healthcare will become Haleon in July, leaving a biopharma-focused ‘New GSK.’ The separation has been planned since early 2020.
At Haleon, you’ll find Sensodyne toothpaste, Panadol and Advil pain medications, and Centrum vitamins.
GlaxoSmithKline’s total operating profit increased by 65% to £2.8bn in Q1 2021, compared to £1.69bn in Q1 2021, which included a £924m upfront payment from Gilead.
GlaxoSmithKline’s total EPS increase 67% to 35.9p compared to 21.5p in Q1 2021 due to leverage from significant sales growth during the quarter such as Gilead.
GlaxoSmithKline will propose a quarterly dividend of 14p, down from 19p in the first quarter of 2021.
GSK expects to pay a 27p dividend in the first half of the year under its new dividend policy, with 22p going to the new GSK and 5p to consumer healthcare, which will soon be Haleon.
Outlook
In terms of the forecast for the remaining firm without the consumer health division, the new GlaxoSmithKline expects sales to expand by 5% to 7% at constant exchange rates in 2022, and adjusted operating profit to grow by 12% to 14% at constant currency, compared to 2021.
GSK stated that the company intends to continue delivering on its strategic priorities.
GlaxoSmithKline expects sales of Specialty Medicines to climb 10% at constant currency this year, while sales of General Medicines show a modest dip, assuming global economies and healthcare systems return to normalcy as the year unfolds.
This was “primarily reflecting increased generalisation” of established Respiratory medications, according to GlaxoSmithKline. Sales of vaccines are likely to climb in the low teens for the entire year.
Covid-19 solutions will generate similar sales in 2021, “but at a substantially reduced profit contribution due to the increased proportion of lower margin Xevudy sales,” according to the pharmaceutical company.
Emma Walmsley, Chief Executive Officer, GSK said, “We have delivered strong first quarter results in this landmark year for GSK, as we separate Consumer Healthcare and start a new period of sustained growth.”
“Our results reflect further good momentum across specialty medicines and vaccines, including the return to strong sales growth for Shingrix and continuing pipeline progress. We also continue to see very good momentum in Consumer Healthcare, demonstrating strong potential of this business ahead of its proposed demerger in July, to become Haleon.”
“This is going to be a landmark year for GSK, with a step-change in growth expected and multiple R&D catalysts, including milestones on up to 7 key late-stage pipeline assets,” said Walmsley after GSK’s FY 21 results.
Glaxo Latest News
In late February, GlaxoSmithKline and Sanofi SA planned to provide data for both the booster and phase three efficacy trials which will be used to support regulatory applications for Covid-19.
In the group’s final analysis of the VAT02 booster, the study revealed that it can raise antibodies 18-to-30-fold across vaccine platforms, including mRNA and adenovirus vaccines.
In addition, two doses of the Sanofi-GSK vaccination in seronegative people exhibited 100% effectiveness against severe Covid-19 illness and hospitalisation in the VAT08 phase three primary series trial.
GlaxoSmithKline and Sanofi’s vaccine has a 75% efficacy rate against moderate or severe Covid-19, and a 58% efficacy rate against any symptomatic Covid-19, which is in line with existing vaccination efficacy expectations, including variations of concern.
Numerous regulatory bodies, including the US Food and Drug Administration and the European Medicines Agency, are currently in discussions with GlaxoSmithKline and Sanofi.
“The evolving epidemiology of Covid-19 demonstrates the need for a variety of vaccines. Our adjuvanted protein-based vaccine candidate uses a well-established approach that has been applied widely to prevent infection with other viruses including pandemic flu. We are confident that this vaccine can play an important role as we continue to address this pandemic and prepare for the post-pandemic period,” said Roger Connor, President of GSK Vaccines.
GlaxoSmithKline at the end of March announced its commencement of clinical development of its second-generation Covid-19 vaccine candidate, CV2CoV, along with CureVac NV, a German biopharmaceutical company.
CureVac and GSK launched their infectious illness collaboration in July 2020, intending to develop new therapeutics based on CureVac’s mRNA technology for a variety of infectious disease targets.
The company said that dosage has been administered to the first participant in a phase one study of the candidate.
The trial will enrol up to 210 adults and will be conducted at clinical sites across the United States to assess the safety, reactogenicity, and immunogenicity of CV2CoV at doses ranging from 2 to 20 micrograms.
The phase one study’s findings are expected in the second half of 2022.
CV2CoV showed faster induction of greater antibody titers, better immunological memory induction, and superior protective efficacy in preclinical research released in November compared to CureVac’s first-generation vaccine candidate, CVnCoV.
Later in March, the US FDA said the emergency use authorisation fact sheet was updated for Sotrovimab which is an experimental Covid-19 neutralising monoclonal antibody.
The FDA assessed that the sotrovimab 500mg dose is unlikely to be effective against the Omicron BA.2 strain, according to GlaxoSmithKline.
GlaxoSmithKline and Vir Biotechnology are putting together a package of evidence to support a higher Sotrovimab dose for the Omicron BA.2 sub-variant, which they will share with regulatory and health authorities around the world for discussion.
Sotrovimab has been given a marketing licence in the European Union as well as a conditional marketing authorization in the United Kingdom and is approved for emergency use in the United States.
Moving into April, GSK announced the acquisition of Sierra Oncology for £1.5bn which complements its commercial and medical expertise in haematology. It will also support the development of its portfolio of new speciality medicines and vaccines.
The group’s application for the review of kidney disease drug daprodustat was accepted by the FDA later in the same month. The drug is used on patients for the treatment of anaemia of chronic kidney disease.
GlaxoSmithKline Pipeline
GlaxoSmithKline partnered up to develop COVID-19 therapeutics and vaccines which have evolved to multiple remedies being in various stages of development, along with two products approved for use.
Monoclonal antibodies
GlaxoSmithKline is collaborating with Vir Biotechnology to analyse and study two monoclonal antibodies that target the COVID-19 virus.
The monoclonal antibodies are generated by cloning an antibody that has been exposed to a certain virus in a lab which enables the production of numerous antibodies that will target the virus.
Monoclonal antibodies act in a similar way to the human immune system and could be a useful tool for people who don’t have a robust natural immune response to a virus.
The FDA granted GlaxoSmithKline Emergency Use Authorization for the first of these monoclonal antibodies in May 2021 and the European Commission approved the same monoclonal antibody for use in the European Union in December 2021.
The second antibody developed in conjunction with Vir is also being studied as a therapy option. A phase 1/2 trial is now being conducted in patients with mild to moderate COVID-19.
Adjuvanted vaccines
GlaxoSmithKline’s partnership with Sanofi is past Phase III and is seeking marketing authorisations.
GlaxoSmithKline and Medicago produced a vaccine using its plant-based technology. The drug was approved for use by Health Canada in February 2022 due to Phase III results.
GlaxoSmithKline and SK Bioscience, which is a South Korean pharmaceutical company, are developing a vaccine which is undergoing Phase III trials.
mRNA COVID-19 vaccines
GlaxoSmithKline and CureVac are developing an mRNA vaccine which will be used to fight against multiple COVID-19 variants. The pre-clinical test results in 2021 were optimistic and the group will begin clinical testing in 2022.
This deal expands on GSK and CureVac’s existing collaboration to produce up to five mRNA-based vaccines and monoclonal antibodies and CureVac has also received a £130 million equity investment from GlaxoSmithKline.
Apart from Covid medication, GlaxoSmithKline has hundreds of treatments on the market and is in the phase of the study to combat problems from dermatology, respiratory, and bacterial infections whilst also providing preventative vaccines for problems such as hepatitis, tetanus, meningitis and more.
Xevudy is a Covid-19 antibody medication developed by GSK in collaboration with Vir Biotechnology, a San Francisco-based immunology business, however, it is no longer allowed for Covid-treatment in any US region due to the surge of the Omicron BA.2 sub-variant.
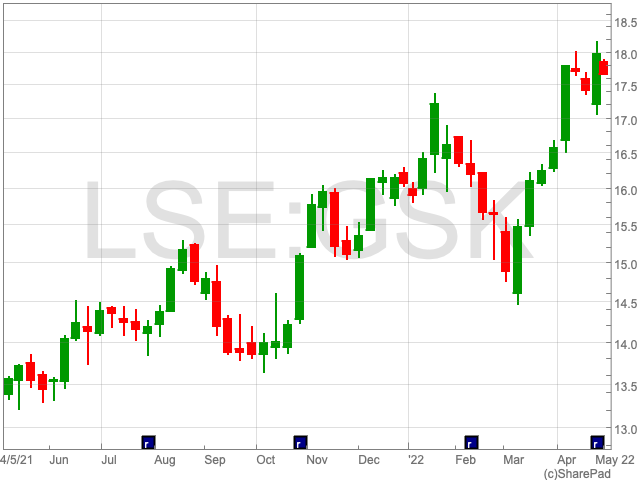
GlaxoSmithKline Share Valuation
GlaxoSmithKline shares peaked at 1,846p in January 2020 and reached its lowest point in 2 years at 1,330p in May 2021 over the last 2 years.
GlaxoSmithKline has a lower forward P/E ratio of 14.8x compared to a trailing P/E ratio of 15.9x, suggesting analysts see GSK’s earnings rising in the next year.
Glaxo’s earnings multiples suggest significant value compared to peer AstraZeneca with a trailing P/E of 25.3x and forward P/E of 19.9x.
Glaxo has a good ROCE of 11.1x compared to Astra’s negative reading.
GlaxoSmithKline can cover its dividend by 1.4x with a yield of 4.5%, which gives investors an indication of expected dividend increases in the future. In GSK’s situation, we can see with a cover of 1.4x, greater dividend payouts in the future are possible, but don’t expect fireworks.
However, the dividend yield is way above the market average meaning the company is good choice for income investors.
In terms of GSK’s broker ratings, Deutsche Bank raised GSK shares to a ‘hold’ and altered its price target from 1,500p to 1,600p. Barclays raised its price target to 1,800p from 1,775p and JPMorgan raised GlaxoSmithKline to a ‘neutral’ rating and increased its target for Glaxo shares to 1,900p from 1,740p.
