The Synairgen share price was up 27.1% to 33.9p in late afternoon trading on Monday, after the biotech group presented the results of its Phase 3 SPRINTER trial for its SNG001 treatment at the American Thoracic Society (ATS) on Monday.
The company reported that its SPRINTER trial failed to meet the primary endpoints of discharge from hospital and recovery, however there was an encouraging sign of reduction in the relative risk of progression to severe disease or death within 35 days.
“The improvement in standard of care for COVID-19 means that most patients are currently discharged fairly rapidly from hospital; however, this further analysis shows that some patients struggle in their battle with the virus and show signs of respiratory compromise, with faster breathing rates and lower oxygen saturations, despite being on oxygen,” said SPRINTER trial chief investigator Tom Wilkinson.
“For these higher-risk patients, there remains an urgent need for new treatment options, and this analysis suggests that SNG001 could be a potentially efficacious treatment option for them.”
Synriagen performed post hoc analysis on a selection of patients including participants over the age of 65, those with co-morbidities linked with worse Covid-19 aftermaths and patients who had clinical signs of compromised respiratory function.
According to the biotech firm, its high-risk patient sub-groups, which made up approximately a third of its participants, responded most positively to its SNG001 inhalable respiratory drug, with the strongest results recorded in those with a compromised respiratory function and SNG001 reportedly reducing the risk of progression to severe disease and death by 70% in the per protocol population.
“The post hoc analyses presented at the ATS conference today suggest that SNG001 may be having a beneficial effect with respect to prevention of severe disease or death,” said Synairgen chief scientific officer Philip Monk.
“These results provide a strong clinical rationale to continue to investigate SNG001 in a trial evaluating progression and/or mortality in hospitalised patients with COVID-19 and more widely in patients with severe viral lung infections.”
However, some analysts commented that the per protocol population was a less reliable metric than the intent-to-treat basis, alongside an “underwhelming” nominal p value of 0.046.
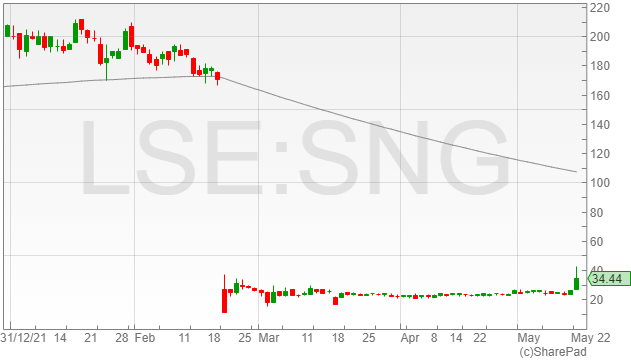
The Synairgen share price is currently trading a far drop below its 177p level from late February 2022, however it has made up some of its lost ground on the back of its results presented today.
The SNG001 drug may not be the golden egg Synairgen was hoping to lay, but perhaps it’s not time to kill the golden goose just yet.
