Exciting AVA6000 RNS could be just the start of a multi-billion-pound bidding war for FTSE AIM minnow.
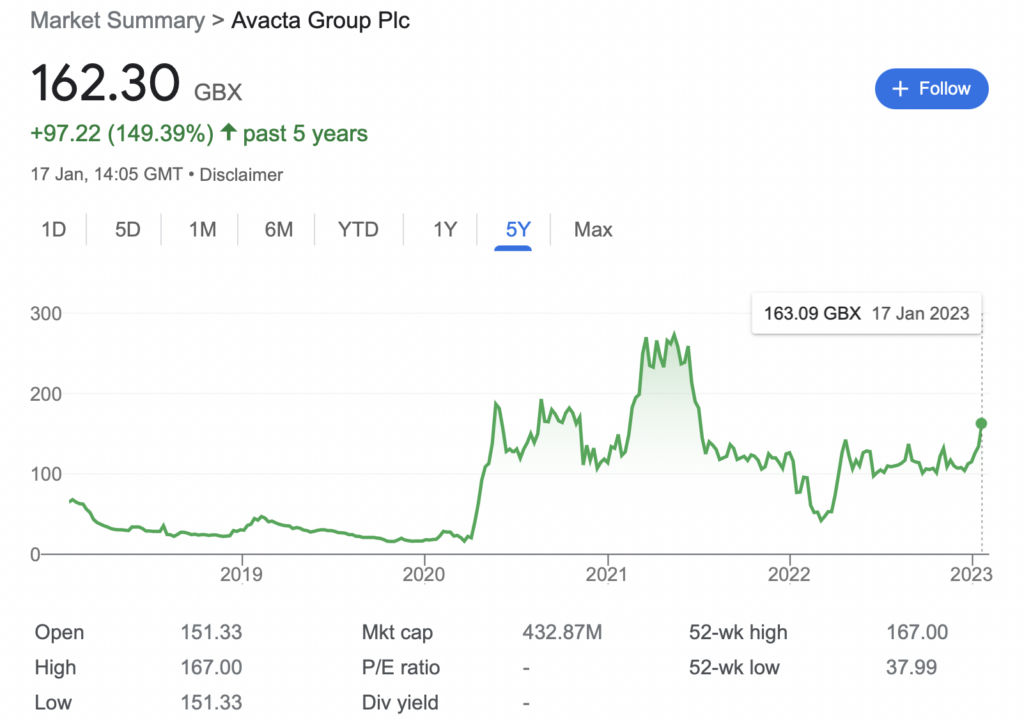
It’s perhaps not controversial to state that long-term investors in Avacta (LON: AVCT) shares have had their faith tested over the past few years. The FTSE AIM biotech has been caught in waves of investor overexuberance and despair — reaching a record 273p during the Reddit-induced meme hysteria of early 2021 — and lows of 42p less than a year ago.
But for small-cap investors looking for the next big thing, nerves of steel and a willingness to wade through paper losses remain the essential pre-requisite to success. And with its latest RNS, their patience is starting to pay off.
Avacta shares: condensed investment case
Avacta has developed two proprietary platforms that it believes could be medicinally revolutionary.
The first, Affimer, has been developed as an alternative to the $100 billion antibody therapy market, and is proposed to eliminate the many negatives of using antibodies to combat disease, including the reliance on the response of an animal’s immune system, their poor specificity with unintended side effects, and the high cost of manufacture.
The second, pre|CISION, is a newly developed chemotherapy platform which only activates within fibroblast activation protein (FAP)-rich tissue which occurs in cancerous tumours. The company hopes that the platform will alleviate many of the unwanted side-effects of chemo.
Financially, Avacta has something in common with smaller biotechs; while revenue increased to £5.5 million in H1 2022, operating losses mounted to £9 million. But hasn’t deterred investors, and indeed, the company has entered multiple partnerships with big players including a joint venture with DaeWoong Pharmaceutical, a licence agreement with Point BioPharma, and a $400 million partnership with LG Chem.
Avacta has ambitions to develop an M&A-led strategy, building a wide defensive moat around its growing position within the European immunodiagnostics markets. As a first step, it has conditionally agreed to acquire IVD distributor Launch Diagnostics for £24 million, with an earn-out capped at £13 million.
Launch should work synergistically with Avacta; three-quarters of its 2021 revenue of £32.75 million was derived within the UK, and the company boasts an impressive gross margin of 44-50%. Moreover, Launch’s covid-related revenue was discounted by 80% as part of the deal, as at the time, it seemed the pandemic was essentially over. With cases rising sharply in China as the country exits lockdown, this is no longer guaranteed.
Promisingly, Avacta is part-funding the takeover by taking an issue of a 6.5% £55 million 5-year, unsecured bond (convertible at 118.75p) to Heights Capital, a subsidiary of titan Susquehanna International. The investing titan does not lend money loosely.

AVA6000: the RNS investors were waiting for
Avacta has multiple treatments in development, but its flagship is undoubtedly pre|CISION platform based AVA6000. The pro-doxorubicin chemotherapy drug saw Phase I trials begin in August 2021, with Avacta hoping to make a serious improvement in the anthracyclines market, which is expected to grow to $1.38 billion by 2024.
For context, AVA6000 has already been granted Orphan Drug Designation by the US Food and Drug Administration. As well as providing tax credits for taking on the risk of developing novel treatments, the designation means Avacta will benefit from seven years of market exclusivity in the US, a benefit described by CEO Alastair Smith as a ‘significant commercial advantage.’
On 17 January, the company finally released a long-awaited AVA6000 RNS entitled ‘Successful Completion of Fourth Dose Escalation.’ It was almost excessively positive, stating that ‘that AVA6000 continues to show a very favourable safety profile in the fourth dose cohort of the ALS-6000-101 dose escalation phase 1 clinical trial.’
Impressively, the company conducted analysis on tumour biopsies procured from six patients that indicated ‘doxorubicin is being released within the tumour tissue confirming the tumour targeting potential of the pre|CISION technology.’
With AVA6000 continuing to be well tolerated, in cohort 4 patients, the company saw a ‘marked reduction’ in the ‘typical toxicities associated with the standard doxorubicin chemotherapy administration.’ These include the well-known adverse side-effects of chemotherapy, including myelosuppression, nausea, vomiting, mucositis, cardiotoxicity, and perhaps most poignantly, hair loss.
The marked reduction specifically in doxorubicin cardiotoxicity, though not quantified, was observed even at double the normal dose, strongly suggesting that the targeting platform is working. Indeed, analysis of tumour biopsies across several cohorts show that ‘AVA6000 targets the release of doxorubicin to the tumour tissue at therapeutic levels which are much higher than the levels being detected in the bloodstream at the same timepoint.’
At present, the UK Safety Monitoring Committee has recommended continuation to higher dose cohorts to establish the maximum tolerated dose, described by CEO Alastair Smith as ‘an unexpected and very positive development’ — of course, investors now already know that at least double the normal Doxorubicin dose is well tolerated.
Smith remains ‘delighted with the very positive data,’ enthusing that ‘the pre|CISION platform has the potential to significantly improve the safety and tolerability of chemotherapies.’ Incredibly, the CEO now envisages the treatment could mean ‘chemotherapy without side effects.’ His words, not mine, and absolutely revolutionary assuming AVA6000 continues to commercialisation.
This is all very promising news, and Avacta’s share price has responded by rising by 17% to 161p (at time of writing). However, it’s worth noting that this is still early days; only 19 patients have been enrolled across all four cohorts so far. There is a long road between now and commercialisation, including Phase 2 and Phase 3, in addition to regulatory approval. This is a risk — and the biotech graveyard is littered with failed advanced phase treatments.
Valuation
Valuing Avacta is an extremely complex task. While investors are rightly focussed on AVA6000, this is only one treatment using the pre|CISION platform, with more than a dozen others in the potential pipeline including AVA3996, its next candidate. Affimer is also exceptionally promising, and potentially worth more than the company’s current market cap alone.
But to value AVA6000: the markets have not reacted fairly to the RNS, with the entire company’s market cap still under £425 million. To big pharma — FTSE 100 giant AstraZeneca, for example — this is little more than pocket change.
As a reminder, Smith told investors as far back as April 2018 that ‘Sanofi’s recent acquisition of a clinical stage comparator to Avacta (Ablynx) for $5 billion highlights the potential valuation of a clinical stage platform technology…with a pipeline of assets in development.’
In fact, AstraZeneca has just completed a $320 million acquisition of Neogene Therapeutics, which specialises in novel T-Cell receptor cancer therapies and is now pursuing a $1.8 billion deal for US-based Cincor. They’re not the only pharma giant on the prowl, as the high interest rate environment both reduces the valuations of speculative biotech stocks and makes investors and scientists alike more amenable to takeovers to fund research.
Importantly, Avacta has announced it plans to hold a Science Day on 23 February, specifically targeted at ‘city analysts and fund managers.’ This will almost certainly be used to provide exact AVA6000 trial details in order to focus the minds of would-be buyers; once the data is in public hands, it will not be long before a bidding war starts — though this is just my opinion.
One curveball that investors may want to consider is the long tenure of Smith. The CEO has led the company for nearly 18 years, and previously worked as a Professor at the University of Leeds. PwC research shows that the average FTSE 100 CEO lasts about five years, with AIM CEOs lasting even less.
It is very possible that Smith will want to continue to hold onto Avacta’s independence, in which case license deals and its history of strategic partnerships will come into play. Note, this is only speculation, but it can be hard for returns-focused investors to remember that this is the man’s scientific Magnum Opus.
But if there is a targeted buyout for Avacta, the price commanded could well be in the billions. The global oncology drugs market was worth circa $141 billion in 2019 and is projected to reach $394 billion by 2027. It’s hard to put a price on chemotherapy without side effects — both financially and in terms of human advancement.
But as outlandish — or not depending on your philosophical perspective — as it sounds, I believe that £3 billion could be a conservative estimate.
This article has been prepared for information purposes only by Charles Archer. It does not constitute advice, and no party accepts any liability for either accuracy or for investing decisions made using the information provided.
Further, it is not intended for distribution to, or use by, any person in any country or jurisdiction where such distribution or use would be contrary to local law or regulation.
Key references:
